Initial assessment of seizure in a postpartum patient should include a broad differential with pregnancy-associated causes and other causes.
Postdural puncture headache and its sequelae should be included in the differential diagnosis of seizure, even in the absence of clinically recognized dural puncture.
Seizure in a patient with a postdural puncture headache is uncommon; early consultation with an anesthesiologist for potential epidural blood patch therapy should be considered.
A 31-year-old woman presented to the emergency department on postpartum day 7 after 4 episodes of generalized tonic–clonic seizures. Her recent pregnancy with her third child was uncomplicated, culminating in a spontaneous vaginal delivery, during which an epidural was placed for analgesia. The epidural insertion required 2 attempts; successful loss of resistance to saline was obtained, with easy insertion of the epidural catheter and no aspiration of cerebrospinal fluid. The patient’s epidural provided asymmetric analgesia with no associated motor blockade, and no concern for dural puncture was documented. The patient had had 2 previous uncomplicated pregnancies delivered under epidural anesthesia.
Twenty-four hours after discontinuation of the epidural, the patient developed a headache that was relieved when she lay supine and was accompanied by dizziness, nausea and vomiting. The features of the headache were consistent with intracranial hypotension and postdural puncture headache (PDPH). The patient had initially tried to treat the headache with acetaminophen, oral hydration and caffeine. However, the symptoms progressively worsened over the following 6 days, and on postpartum day 7 she had seizures at home and during transport to the emergency department.
In the emergency department, the patient was tearful, drowsy and somewhat confused, consistent with a postictal state. Her blood pressure was 109/72 mm Hg and her pulse was 93 beats/min. Her body mass index was 26. Her neurologic examination — including cranial nerves, strength, reflexes and sensory examination — was normal. Her neck was supple with no evidence of meningeal irritation. Examination of the spine and the site of epidural insertion did not reveal any skin changes, tenderness or signs of infection. The patient had history of mild hypothyroidism and gastroesophageal reflux disease; she had no history of hypertensive disorders of pregnancy.
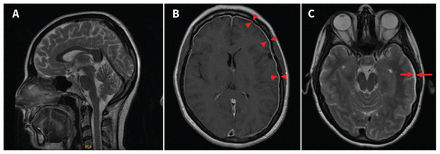
We administered magnesium sulfate for seizure prophylaxis (4 g intravenously over 15 min, followed by a 1 g/h infusion over the next 24 h). We considered many differential diagnoses, particularly eclampsia, new primary seizure disorder, intracranial hemorrhage, infection of the central nervous system, intracranial mass and late presentation of an epidural complication (Box 1). Complete blood count, serum creatinine, aspartate aminotransferase, alanine aminotransferase, international normalized ratio (INR) and partial thromboplastin time (PTT) were normal, and urinalysis was negative for protein; we therefore thought that eclampsia was unlikely. A computed tomography (CT) scan of the patient’s head without contrast showed findings suggestive of intracranial hypotension secondary to spinal puncture, including a left subdural hygroma (Figure 1). A CT scan of her head with contrast showed no evidence of dural venous sinus thrombosis. Magnetic resonance imaging (MRI) showed findings consistent with intracranial hypotension and showed the left subdural hygroma (Figure 2).
Box 1: Differential diagnosis for postpartum seizure
Obstetric
Postpartum eclampsia
Sheehan syndrome
Amniotic fluid embolism
Anesthestic
Postdural puncture headache
Air embolism
Neurologic
Primary seizure disorder
Structural lesion
Stroke
Intracranial hemorrhage
Posterior reversible encephalopathy syndrome
Reversible cerebral vasoconstrictor syndrome
Spontaneous leak of cerebrospinal fluid
Metabolic
Withdrawal syndrome (e.g., alcohol withdrawal)
Electrolyte abnormalities
Uremia
Hypoglycemia
Thyroid abnormalities
Infectious
Meningitis or encephalitis
Psychiatric
Pseudoseizure
Other
Porphyria
Unenhanced computed tomography brain scans of a 31-year-old woman with postpartum seizures. (A) A sagittal view showed effacement of the pre-pontine cistern (arrow pointing to the right) with mild sagging of the brainstem. Cerebellar tonsils are normal with no ectopia or herniation below the foramen magnum (arrow pointing to the left). Axial views showed (B) effacement of the basal cisterns and (C) a small, left subdural hygroma (arrows) with mild local mass effect, resulting in sulcal effacement.
T2-weighted magnetic resonance imaging brain scans of a 31-year-old woman with postpartum seizures. (A) Midline sagittal view showing no pituitary enlargement. (B) Axial views showing diffuse smooth and linear dural or pachymeningeal enhancement along the cerebral convexities (arrowheads) — a finding of intracranial hypotension — and (C) T2 hyperintense subdural hygroma (arrows).
We admitted the patient to the internal medicine service and consulted an obstetrician, anesthesiologist, neurologist and neurosurgeon. The neurosurgeon considered there was no role for surgical intervention. Given the convincing symptoms for PDPH and findings suggestive of intracranial hypotension on imaging, the anesthesiologist performed an epidural blood patch 1 day after admission. When 28 mL of blood had been placed into the epidural space, the patient reported pressure in the lower back and immediate relief of the headache. Before discharge, we ordered an electroencephalogram (EEG) to assess for a primary seizure disorder; no epileptiform abnormalities were found. The patient did not have any further seizures and was discharged 5 days after admission. Because a reversible cause of her seizures had been identified, the neurologist decided against prescribing antiepileptic medication. Two months later, a follow-up MRI was normal, with resolution of the subdural collection.
Discussion
Seizure in a postpartum patient can be a diagnostic challenge; clinicians must consider both pregnancy-specific causes and other causes (Box 1). This case highlights that dural puncture with intracranial hypotension, although uncommon, should be considered as a cause of seizure after epidural analgesia. The combination of postpartum headache and seizure should raise concern for postpartum eclampsia, even in the absence of other common symptoms such as visual changes, edema and epigastric pain.1 Normotensive eclampsia has been reported but is uncommon. 2 Another potential cause of postpartum seizure and headache is reversible cerebral vasoconstriction syndrome, characterized by reversible, multifocal vasoconstriction of the large-and medium-sized cerebral arteries. The qualities of the headache in reversible cerebral vasoconstriction syndrome are similar to that of eclampsia, with a severe, diffuse, “thunderclap” quality.3 This is in contrast to the headache associated with PDPH, which is positional (i.e., worse when upright and relieved when lying supine) and is often accompanied by photophobia, nausea or vomiting.4
Initial workup for seizure in pregnant and postpartum patients should include investigations of complete blood count, liver enzymes, creatinine, fibrinogen, PTT, INR, urinalysis or albumin–creatinine ratio, electrolytes, blood glucose, calcium and magnesium. These investigations can assist in identifying preeclampsia and HELLP syndrome (hemolysis, elevated liver enzymes, low platelets). If these initial investigations are normal, eclampsia-induced seizure is less likely, particularly if the patient is normotensive. At this point, alternate causes should be considered, and specialist input sought, as indicated.
Initial imaging of the brain usually consists of CT with contrast or MRI, which can identify intracranial pathology such as intracranial hemorrhage, infarction or structural lesions. Intracranial hypotension is diagnosed by characteristic changes on MRI (i.e., presence of subdural collections, pachymeningeal enhancement or sagging of the brain) and presence of at least 1 of the following features: opening pressure of less than 60 mm H2O, spinal meningeal diverticulum on MRI or improvement of symptoms after epidural blood patching.5 An increased height of the pituitary gland on MRI has been reported in patients with symptomatic intracranial hypotension; a height above the normal limit has a sensitivity of 63% and specificity of 97%.6 Interestingly, the pituitary size was not increased in our patient (Figure 2A). Intracranial hypotension leads to sagging of the brain and tension on veins between the dura and arachnoid. If these vessels rupture, a subdural hygroma can form, as in this patient. If CT and MRI of the head do not show a cause of seizures, a magnetic resonance angiogram to assess for cerebral artery narrowing, as is seen in reversible cerebral vasoconstriction syndrome, should be considered.3 An EEG can identify a primary seizure disorder; patients with epilepsy are prone to postpartum seizures due to sleep deprivation.
If the patient is actively seizing, treatment should focus on termination of the seizure; magnesium sulfate is the first-line agent if eclampsia is the presumed cause. Magnesium sulfate can be infused for further seizure prophylaxis. Patients’ reflexes and respiratory rate should be monitored closely given the risk of toxicity and respiratory depression from magnesium, which occurred in about 1% of patients allocated magnesium sulfate and 0.5% allocated placebo in 1 review.7 This risk is usually outweighed by a 58% relative risk reduction in eclamptic seizure among patients with preeclampsia treated with magnesium sulfate (number needed to treat 100 to prevent 1 seizure).7
Once it has been determined that the patient has PDPH-associated seizure, an epidural blood patch should be considered. The procedure involves injection of about 20 mL of autologous blood (obtained through a venous blood sample) into the epidural space. The success rate is variable, with complete or partial relief reported in 50%–80% of cases.8 Injection of more than 20 mL has not been consistently shown to provide greater relief; however, standard of care in some hospitals is to inject until the patient feels fullness or discomfort in the lower back, which can occur when slightly less or slightly more than 20 mL has been injected.8 The procedure carries a small risk of bleeding and infection. About 50% of patients have back pain 24 hours after the procedure, but no evidence suggests an increased frequency of chronic back pain.9 Two main theories have been proposed to explain how an epidural blood patch works. The plug theory suggests that the blood placed in the epidural space forms a fibrinous clot that seals the dural hole, preventing further leakage of cerebrospinal fluid (CSF).9 However, this fails to explain why patients feel immediate relief after the procedure. The pressure patch theory suggests that blood injected into the epidural space elevates the pressure in the subarachnoid space by compressing the dura instantaneously, causing CSF to migrate upwards and immediately restore intracranial CSF volume and pressure.9 The true mechanism is likely a combination of the 2 theories.
The incidence of PDPH after spinal anesthesia has been estimated to be 6%–36%.10 However, its prevalence after epidural catheterization without evidence of dural puncture has not been described, despite the widespread use of epidural analgesia. 10 The incidence of PDPH among patients after epidural-induced dural puncture is reported to be 76%–85%.11 Importantly, postpartum patients are at particularly high risk for PDPH given that female gender, young age and pregnancy are all independent risk factors for the development of PDPH.10 Patients with a history of PDPH have an increased incidence of postpartum depression, post-traumatic stress disorder, chronic headache and backache, and decreased breastfeeding at 6 months after the epidural.12
The mechanisms underlying PDPH are uncertain. Proposed mechanisms include traction on the pain-sensitive structures within the intracranial space as a result of CSF volume loss and subsequent compensatory vasodilation of intracranial vessels in an attempt to maintain intracranial volume.4 Inadvertent puncture of the dura during epidural insertion leads to a larger loss of CSF volume compared with spinal anesthesia because of the larger bore needles used. The mechanism underlying seizures after PDPH is also uncertain. However, using arteriography, Shearer and colleagues13 showed that a possible mechanism includes regional blood flow changes from diffuse vasospasm. This vasospasm may be secondary to anatomic brain displacement associated with loss of CSF volume.
The development of late seizure after PDPH is an uncommon complication. Given the broad differential and possible complications in a postpartum patient presenting with seizure, diagnostic workup should be timely and comprehensive, and management should involve a multidisciplinary team.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Ejaz Causer and Isabelle Birchall drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Ejaz Causer and Isabelle Birchall are joint first authors.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/