Patent foramen ovale is usually an incidental finding but can sometimes cause hypoxemia.
Patent foramen ovale should be considered when the degree of hypoxemia is disproportionate to the underlying lung pathology.
Elevated right atrial pressure is not always necessary for right-to-left shunting in patients with patent foramen ovale.
Percutaneous closure has become a safe and effective treatment for hypoxemia associated with patent foramen ovale.
A 65-year-old woman presented to hospital with a weeklong history of progressive, profound difficulty breathing and decreased tolerance to exercise. The patient had stage 4 high-grade neuroendocrine carcinoma of unknown primary with liver metastases for which she was receiving chemotherapy with carbo platin and etoposide. She did not have fever, chills, cough or chest pain, was not coughing up blood, and did not experience shortness of breath while lying down.
On physical examination, the patient’s temperature was 36.7°C, her blood pressure was 107/68 mm Hg, her heart rate was 62/min and her respiratory rate was 20 breaths/min. Oxygen saturation was 93% breathing 5 L/min oxygen by nasal cannula. She was in mild respiratory distress. A cardiac examination showed a regular rhythm with normal S1 and S2, and a grade 3 systolic murmur best appreciated at the left lower sternal border. No jugular venous distention was seen. The lungs were clear, and there was no peripheral edema or signs of deep vein thrombosis.
Laboratory investigations showed normal white blood cell count, platelets, electrolytes, creatinine and international normalized ratio. Hemoglobin was stable in the 90s. Troponin was elevated at 28 (normal ≤ 14) ng/L. An electrocardiogram showed T wave inversions in anterior leads. Radiography of the chest was normal, but computed tomographic (CT) pulmonary angiography showed multiple bilateral subsegmental pulmonary emboli, likely from the underlying cancer. The patient was admitted to hospital and given therapeutic anticoagulation with dalteparin.
The following day, the patient’s hypoxemia worsened, requiring high-flow oxygen at 40 L/min to maintain saturation above 90%. Over the next several days, we continued to give her anticoagulation for pulmonary embolism, but her hypoxemia persisted. We repeated CT pulmonary angiography, which showed resolution of the pulmonary emboli; however, she still required high-flow oxygen. Given these unexpectedly high oxygen requirements, we ordered transthoracic echocardiography, which showed a left ventricular ejection fraction of 50%, severe tricuspid regurgitation (Figure 1; Video 1 [Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.180243/-/DC1]), right ventricular volume overload and right ventricular systolic pressure of 38 mm Hg. The pulmonic valve was not visualized. The tricuspid leaflet had thickening consistent with carcinoid heart disease. A patent foramen ovale was noted on transthoracic echocardiography with contrast (Figure 2; Video 2 [Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.180243/-/DC2]).
Transthoracic echocardiogram with colour doppler in apical four-chamber view showing severe tricuspid regurgitation (arrow) in a 65-year-old woman with carcinoid heart disease and patent foramen ovale. Note: LA = left atrium, LV = left ventricle, RA = right atrium, RV = right ventricle.
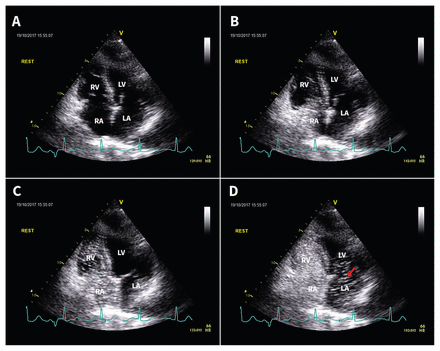
Transthoracic echocardiogram (i.e., a bubble study) in apical four-chamber view showing right-to-left shunt through a patent foramen ovale. The agitated saline can be seen entering the right atrium (, A and B), right ventricle (C), and left atrium (arrow, D) within a single cardiac cycle. Note: LA = left atrium, LV = left ventricle, RA = right atrium, RV = right ventricle.
The patient’s hypoxemia eventually improved, and oxygen was weaned down to 3 L/min. We thought the patent foramen ovale was an incidental finding and attributed her hypoxemia to pulmonary embolism. We considered suggesting palliative care. However, ultimately, home oxygen was arranged, and the patient was discharged without further intervention.
The patient returned to hospital two months later with severe hypoxemia, in addition to having had a brief transient ischemic attack that resulted in slurred speech and left arm weakness. This time, left and right heart catheterizations were performed (Boxes 1 and 2). Pulmonary artery pressure was normal and right atrial pressure was not substantially elevated. A tricuspid regurgitant jet was streaming directly across the patent foramen ovale (Figure 3; Video 3 [Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.180243/-/DC3]). The direction of the jet made right-to-left shunt possible without a pressure gradient between the two atria.
| Pressure, mm Hg | ||||
|---|---|---|---|---|
| Anatomy | Systolic | Diastolic | End-diastolic | Mean |
| Right atrium | 9* (−1 to 8) | |||
| Right ventricle | 32 (15 to 28) | 12 (0 to 8) | ||
| Pulmonary artery | 22 (15 to 28) | 9 (5 to 16) | 15 (10 to 22) | |
| Pulmonary capillary wedge | 11 (6 to 15) | |||
| Left atrium | 10 (4 to 12) | |||
| Left ventricle | 128 (90 to 140) | 11 (4 to 12) | ||
Note: Normal ranges are provided in parentheses for reference.
↵* Right atrial pressure is minimally above normal, but remains lower than left atrial pressure.
| Right atrium | 31.7% |
| Pulmonary artery | 31.2% |
| Pulmonary veins | Left upper: 99.9% |
| Left lower: 97.3% | |
| Right upper: 99.3% | |
| Right lower: 99.5% | |
| Aorta | 69.6% |
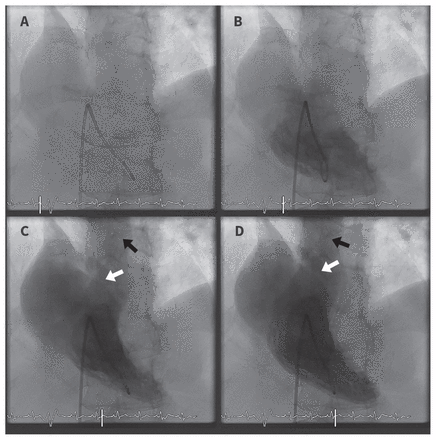
Right ventricular angiogram from right heart catheterization showing right-to-left shunt through a patent foramen ovale. At the beginning, contrast was injected into the right ventricle (A and B). During ventricular systole, two streams of blood flow can be seen originating from the right ventricle, with one in the pulmonary trunk (C and D, black arrow), and the other through the patent foramen ovale (C and D, white arrow).
The patient underwent percutaneous closure of the patent foramen ovale, and her oxygen saturation immediately corrected to 96% on room air. She was discharged home the next day in stable condition and continues to do well while still receiving chemotherapy four months later.
Discussion
This patient’s case shows the challenge for clinicians in recognizing the uncommon circumstance when a patent foramen ovale may be the underlying cause of a patient’s hypoxemia. Our patient returned to hospital with persistent hypoxemia inadequately explained by lung pathology on imaging, and hence intracardiac right-to-left shunt was a major diagnostic consideration. Right heart catheterization was pursued, and angiography of the inferior vena cava showed evidence of venous blood being redirected to the patent foramen ovale by the tricuspid regurgitant jet. The severe tricuspid regurgitation in this patient was a result of carcinoid heart disease. Pulmonary and right atrial pressures were relatively normal, ruling out pulmonary embolism as a cause of the shunt. Poor compliance of right ventricle from severe tricuspid regurgitation likely worsened the shunt.
Correct diagnosis led to definitive management of the patient’s hypoxemia with closure of the patent foramen ovale. Despite the patient’s advanced cancer, the procedure improved her quality of life and allowed her to continue receiving chemotherapy. In addition, she was protected from future stroke or transient ischemic attack caused by paradoxical embolism.
Carcinoid heart disease
Neuroendocrine tumours are uncommon, usually developing from neuroendocrine cells in the gastrointestinal tract (67.5%) or bronchopulmonary system (25.3%).1 These tumours may secrete vasoactive substances, including serotonin, tachykinins, kallikrein and prostaglandins that are typically degraded and inactivated by the liver.1 If a primary gastrointestinal neuroendocrine tumour metastasizes to the liver, these substances may enter systemic circulation and result in carcinoid syndrome, characterized by flushing, diarrhea, bronchospasm and hypotension.1 Vasoactive peptides are thought to mediate fibrous plaque deposition on right-sided heart valves, leading to tricuspid and pulmonary regurgitation and, less frequently, tricuspid and pulmonary stenosis.1 In a cohort study that included patients with carcinoid heart disease, tricuspid regurgitation was present in all patients, and was moderate or severe in 90% of patients.2
Even metastatic high-grade neuroendocrine carcinoma is responsive to chemotherapy; with platinum agents plus etoposide chemotherapy, median survival is between 10 and 14 months.3
Patent foramen ovale
Patent foramen ovale is a congenital cardiac lesion that results from incomplete closure of the foramen ovale that persists into adulthood.4 A large autopsy study of found that patent foramen ovale is present in about 27% of the population.4 Most patent foramen ovale are asymptomatic, but they can sometimes cause serious clinical consequences, including paradoxical embolism, decompression sickness in scuba divers and platypnea–orthodeoxia syndrome.4,5
Transthoracic echocardiography with contrast (i.e., a bubble study) is considered the initial imaging modality of choice for diagnosing patent foramen ovale.6 A test is considered positive when one or more bubbles are seen in the left atrium within three cardiac cycles.6 Bubbles entering the left atrium after three cardiac cycles suggests intrapulmonary shunt.6 The sensitivity of detecting patent foramen ovale on echocardiography can be improved by provocative maneuvres such as releasing a sustained Valsalva or cough.6 Although transesophageal echocardiography is more sensitive than transthoracic echocardiography, clinicians need to consider the invasive nature and the higher risk of complications when ordering a transesophageal echocardiography.6 This is particularly important when considering that most patent foramen ovale are not clinically relevant.
Patent foramen ovale and hypoxemia
Hypoxemia associated with patent foramen ovale typically manifests as platypnea–orthodeoxia syndrome,5 an uncommon phenomenon whereby patients have dyspnea (platypnea) and arterial oxygen desaturation that worsens when the patient is in the upright position (orthodeoxia).5 Both cardiac and noncardiac causes of platypnea–orthodeoxia syndrome exist.5
For cardiac platypnea–orthodeoxia syndrome to occur, two components are required: an anatomic and a functional defect.7 An anatomic defect is any communication between the two atria, such as patent foramen ovale.7 This alone does not lead to right-to-left shunting owing to higher systemic circulation pressures.7 Blood cannot flow against this pressure gradient, hence patent foramen ovale do not generally result in hypoxemia.
A functional defect can precipitate the shunt in a patient with a pre-existing patent foramen ovale by elevation of right atrial pressures that result in a reversal of the interatrial pressure gradient.8 This reversal is associated with conditions that either increase pulmonary artery pressure (e.g., pulmonary embolism, pulmonary hypertension, pneumonectomy) or increase right-sided filling pressures (e.g., constrictive pericarditis, pericardial effusion).8
A second mechanism involves venous blood return being preferentially redirected toward the patent foramen ovale.8 Normally, venous blood entering the right atrium is not aimed toward the atrial septum.8 However, certain functional defects such as prominent eustachian valve (valve of the inferior vena cava), aortic abnormalities and atrial septal aneurysm can alter this normal pattern of blood flow.8 In our patient’s case, tricuspid regurgitation projected deoxygenated blood toward the patent foramen ovale. Because this is a flow-driven mechanism, right-to-left shunt occurred in the absence of elevated right atrial pressure.
Hypoxemia from patent foramen ovale is uncommon and not well described in the literature. A recently published case report by van der Veer and colleagues described a woman with platypnea–orthodeoxia syndrome caused by posture-dependent dilatation of the ascending aorta and right atrial compression causing preferential blood flow through the patent foramen ovale.9
With small shunts, patients may not experience major dyspnea.5 Occasionally, intracardiac shunt may be small but worsen in the upright position, resulting in platypnea and orthodeoxia.5 However, if the shunt is large, patients will have hypoxemia regardless of posture, as in our patient’s case.5
Management of patent foramen ovale
Most patent foramen ovale are not clinically relevant and do not require intervention. If right-to-left shunt occurs across a patent foramen ovale, definitive management of hypoxemia involves closure of the defect.7 Before proceeding with closure of the patent foramen ovale, oxygen saturation in all four pulmonary veins should be measured to exclude lung pathology as the cause of a patient’s hypoxemia.10 For our patient, all of the pulmonary veins had oxygen saturations above 97% on room air, ruling out pulmonary embolism as the cause of her hypoxemia.
With emerging technologies in recent years, percutaneous closure has largely replaced open heart surgery as first-line treatment of patent foramen ovale.8 Several large case series have shown the efficacy and safety of percutaneous closure for the treatment of hypoxemia associated with patent foramen ovale.10 A recent case series by Shah and colleagues showed that all 52 patients with hypoxemia had improved oxygen saturation immediately after percutaneous closure of their patent foramen ovale.10
Conclusion
Our patient’s case shows the importance of considering a diagnosis of right-to-left shunt from patent foramen ovale when the degree of hypoxemia is disproportionate to underlying lung pathology. With percutaneous therapy becoming more technically feasible and accessible in many Canadian centres, recognizing this entity has considerable therapeutic implications.
Please see the following videos online:
Video 1. Transthoracic echocardiogram with colour doppler in apical four-chamber view showing severe tricuspid regurgitation (www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.180243/-/DC1).
Video 2. Bubble study in apical four-chamber view showing right-to-left shunt through patent foramen ovale (www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.180243/-/DC2).
Video 3. Right ventricular angiogram from right heart catheterization showing right-to-left shunt through a patent foramen ovale (www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.180243/-/DC3).
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: Chengyue Yang and Aditya Sharma conceived and designed the manuscript. Chengyue Yang drafted the manuscript. Aditya Sharma critically revised the manuscript for important intellectual content. Both of the authors approved the final version to be published and agreed to be accountable for all aspects of the article.