Abstract
Background: Randomized trial evidence suggests that some antiviral drugs are effective in patients with COVID-19. However, the comparative effectiveness of antiviral drugs in nonsevere COVID-19 is unclear.
Methods: We searched the Epistemonikos COVID-19 L·OVE (Living Overview of Evidence) database for randomized trials comparing antiviral treatments, standard care or placebo in adult patients with nonsevere COVID-19 up to Apr. 25, 2022. Reviewers extracted data and assessed risk of bias. We performed a frequentist network meta-analysis and assessed the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Results: We identified 41 trials, which included 18 568 patients. Compared with standard care or placebo, molnupiravir and nirmatrelvir–ritonavir each reduced risk of death with moderate certainty (10.9 fewer deaths per 1000, 95% confidence interval [CI] 12.6 to 4.5 fewer for molnupiravir; 11.7 fewer deaths per 1000, 95% CI 13.1 fewer to 2.6 more). Compared with molnupiravir, nirmatrelvir–ritonavir probably reduced risk of hospital admission (27.8 fewer admissions per 1000, 95% CI 32.8 to 18.3 fewer; moderate certainty). Remdesivir probably has no effect on risk of death, but may reduce hospital admissions (39.1 fewer admissions per 1000, 95% CI 48.7 to 13.7 fewer; low certainty).
Interpretation: Molnupiravir and nirmatrelvir–ritonavir probably reduce risk of hospital admissions and death among patients with nonsevere COVID-19. Nirmatrelvir–ritonavir is probably more effective than molnupiravir for reducing risk of hospital admissions. Most trials were conducted with unvaccinated patients, before the emergence of the Omicron variant; the effectiveness of these drugs must thus be tested among vaccinated patients and against newer variants.
Most trials addressing the treatment of patients with COVID-19 have targeted patients admitted to hospital with severe or critical disease.1 However, more recently, several treatments, including antiviral drugs, antidepressants, monoclonal antibodies and inhaled corticosteroids, have been studied for patients with nonsevere COVID-19.2 Preliminary evidence from ongoing or recently completed trials suggests that 2 novel antiviral drugs — molnupiravir and nirmatrelvir–ritonavir (Paxlovid) — may be effective at reducing risk of hospital admission.3–5 To date, evidence on antiviral drugs for nonsevere COVID-19 has not been systematically synthesized or appraised. Furthermore, although efficacy data from trials of molnupiravir, nirmatrelvir–ritonavir and remdesivir are promising, no head-to-head trials have compared these drugs.
A network meta-analysis allows for comparison of treatments that have not been compared in randomized controlled trials (RCTs), using pooled estimates from direct and indirect evidence. They can provide guidance to clinicians and evidence users in determining which treatments are superior. This is particularly important as health care systems attempt to prioritize access to effective COVID-19 treatments in the early stages of the disease.
We sought to compare the effectiveness of antiviral drugs for patients with nonsevere COVID-19.
Methods
We conducted a systematic review and network meta-analysis, that included a rigorous appraisal of the evidence. We registered a protocol on Open Science Framework and uploaded the data used for this analysis (https://osf.io/zbcf9). We report our systematic review and network meta-analysis in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) extension statement for reporting of systematic reviews incorporating network meta-analyses.6
Search strategy
We worked with an experienced medical librarian to develop a search strategy. We searched for eligible trials using the Epistemonikos COVID-19 L·OVE (Living Overview of Evidence) database and the Cochrane COVID-19 study register, an open-access repository for COVID-19 literature, with a valid search up to Apr. 25, 2022.7
The Epistemonikos COVID-19 L·OVE database is a comprehensive repository that is used as the primary source for several international evidence synthesis initiatives addressing COVID-19,8 and has been validated as a comprehensive source for COVID-19 studies. Two studies validated its reliability as a primary source, identifying 93% of relevant articles in 1 study and 99.67% in another (100% for RCTs).9,10 This repository draws from 41 databases that are updated on a daily to weekly basis, including MEDLINE, Embase, the Cochrane Register, Clinicaltrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform and MedRxiv.
We used the Epistemonikos user interface to identify articles addressing antiviral treatments for COVID-19. We did not use the RCT filter and opted to screen all studies of antiviral drugs ourselves. The articles selected by the interface are first identified using automated tools and then reviewed by methods experts and other members of the Epistemonikos team. We supplemented our search by reviewing 2 large living systematic reviews and network meta-analyses.2,8,11 Appendix 1, Supplement 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220471/tab-related-content presents the search strategy and more details on the Epistemonikos COVID-19 L·OVE interface. Similarly, we used the Cochrane COVID-19 study register interface to search broadly for antiviral drugs used in COVID-19 treatment.
Study selection
We included published and unpublished trials that randomized adult patients (aged ≥ 18 yr) with nonsevere COVID-19 to antiviral treatments, standard care or placebo.
We used the WHO definitions for disease severity;12 patients were classified as having nonsevere disease if they were symptomatic and did not have evidence of lower respiratory disease, moderate disease if they had an oxygen saturation of 93% or more on room air and evidence of lower respiratory disease, and severe or critical disease if they had fever or suspected infection, cough, respiratory rate above 30 breaths/min, severe respiratory distress and oxygen saturation less than 93% on room air.
For trials that reported on patients with differing levels of severity, we extracted data on nonsevere patients, when reported. We included trials that did not specifically report data on nonsevere patients if most of the patients (> 80%) had nonsevere disease. We excluded trials that reported only on patients with severe disease or critically ill patients and those that compared interventions that are not antiviral drugs.
After training and calibration exercises to ensure sufficient agreement, reviewers worked independently and in duplicate to screen titles and abstracts of search records and, subsequently, the full texts of records deemed potentially eligible after title and abstract screening. Reviewers resolved discrepancies by discussion or, when necessary, by adjudication with a third reviewer.
Data collection
After training and calibration exercises to ensure sufficient agreement, reviewers worked independently and in duplicate to extract data from each eligible trial. We collected data on trial characteristics (i.e., author, year published, trial registration and country of enrolment), patient characteristics (i.e., age, sex, inpatient or outpatient, disease severity and comorbidities) and our outcomes of interest. When reported, we also extracted outcome data stratified by age and sex to facilitate subgroup analyses.
Our outcomes of interest were all-cause mortality, hospital admissions, need for mechanical ventilation and serious adverse events leading to stopping the drug — all at longest reported follow-up.
Risks of bias
Reviewers, working independently and in duplicate, assessed risk of bias using a revision of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2.0).13,14 We rated risk of bias as low, probably low, probably high or high, across the following domains: bias arising from the randomization process, departures from the intended intervention, missing outcome data, measurement of the outcome and selection of the reported results. We resolved discrepancies by discussion and, when necessary, with adjudication by a third party. Appendix 1, Supplement 2 presents more details on our risk of bias assessments.
Statistical analysis
For each outcome, we conducted frequentist random-effects network meta-analysis using the restricted maximum likelihood estimator with the netmeta package in R (version 4.03, R Foundation for Statistical Computing).15 The restricted maximum likelihood estimator is used to estimate τ, a measure of heterogeneity in the meta-analysis. The network meta-analysis uses the relative risk (RR) as the measure of treatment effect. We used the total number of patients in each arm and the events for each outcome to calculate the RR and associated 95% confidence interval (CI) using the pairwise function. 16 When there were 0 events reported for both arms, we used the continuity correction and added 0.5 to the event and total numbers. 17 A network meta-analysis produces network estimates from the pooled results of both direct (pairwise, conventional meta-analysis) and indirect evidence (drug treatments with common comparators). This results in more precise estimates, increasing our certainty in the treatment effect. We categorized each antiviral drug separately, and grouped standard care and placebo together.
We used the treatment-splitting method (difference between direct and indirect evidence in closed loops) to test for local incoherence. 18 We assessed heterogeneity in the data by inspection of forest plots and the I2 statistic. We considered heterogeneity ranging from 0%–40% as potentially unimportant, 30%–60% as moderate, 50%–90% as substantial and 75%–100% as critical.19 For comparisons with 10 or more trials, we planned to assess publication bias by visual inspection of funnel plots and Egger’s statistical test.20
For visual presentation, we generated network and forest plots using the network map command in Stata v.17 (StataCorp). In addition to RRs, we summarized intervention effects using the absolute risk difference per 1000 patients, with a baseline risk sourced from the median risk in the placebo and standard care arms across trials. For adverse events that led to stopping the drug, we used the baseline risk in the standard care arms alone.
For comparisons with moderate- or high-certainty evidence, we tested 4 prespecified effect modifiers (age, sex, risk of bias, disease severity) using univariate metaregression models, and judged the credibility of any subgroup effects using the Instrument to Assess the Credibility of Effect Modification Analyses (ICEMAN) tool.21 Within-trial subgroups were preferable to between-trial subgroups because of potential differences between trials that could confound between-trial subgroups.21,22
Assessment of certainty of the evidence
Reviewers, working independently and in duplicate, assessed the certainty of the evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach for network meta-analysis.23,24 We rated the certainty for each comparison and outcome as high, moderate, low or very low, based on considerations of risk of bias, inconsistency, indirectness, publication bias, intransitivity (the dissimilarity of important factors that may affect the outcome being investigated across comparisons), incoherence (difference between direct and indirect effects) and imprecision. Appendix 1, Supplement 3 presents details on methods for assessing the certainty of evidence.
We used a minimally contextualized approach for judgments of imprecision, which considers only whether CIs include a minimally important effect and does not consider the magnitude of plausible effects.25 This approach does not consider statistical significance as the only indicator of whether an intervention is effective, given the important limitations of statistical significance.26 An estimate may not be statistically significant but may still have evidence of moderate certainty for benefit or harm, depending on the width of the CIs and whether they cross the bounds of the prespecified thresholds. Conversely, an intervention may produce results that are statistically significant but that indicate no important benefit or harm (e.g., a < 1% reduction in risk of death).
Based on a survey of the authors, we considered a minimally important effect to be a 1% reduction in risk of death, mechanical ventilation and hospital admission, and a 2% reduction in risk of adverse events that led to stopping the drug.
We report our results using guidance from the GRADE Working Group, which involves describing the effect of a drug based on the certainty of evidence (i.e., high-certainty evidence the drug is effective, moderate-certainty evidence the drug is effective, low-certainty evidence the drug is effective and very low–certainty evidence the drug is effective).27
Ethics approval
We did not seek ethics approval for this systematic review and meta-analysis because the data were publicly available.
Results
Our search identified 4541 unique references. At the title and abstract screening stage, we screened 4475 citations and identified 66 RCTs for full text review (Figure 1). We excluded 25 trials in which all or most patients had severe or critical COVID-19. We sourced data from 1 trial from a press release (EPIC-SR)3 and 1 trial from a large living network meta-analysis.2 We identified 41 eligible trials, with 18 568 patients. Appendix 1, Supplement 4 presents more details on the excluded studies.
Flow diagram. Appendix 1, Supplement 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220471/tab-related-content, presents details on excluded trials.
We identified trials that reported on 16 unique antiviral treatments, including nirmatrelvir–ritonavir, molnupiravir, remdesivir, azvudine, emtricitabine–tenofovir, favipiravir, lopinavir–ritonavir, lopinavir–ritonavir–ribavirin, resveratrol, ribavirin, ribavirin–sofosbuvir–daclatasvir, sofosbuvir–daclatasvir, sofosbuvir–ledipasvir, tenofovir, triazavirin and umifenovir.
Most patients were aged between 36.5 to 65.5 years, with a similar proportion of male to female patients. Although we included 4 trials that included patients with severe COVID-19, we only extracted data from the nonsevere subgroup. The most common comorbidity was hypertension. Table 1 and Appendix 1, Supplement 5 present trial characteristics.28–68
Characteristics of included randomized controlled trials
Risk of bias
Figure 2 presents our risk of bias assessments for the studies that reported deaths. Appendix 1, Supplement 6 presents more details on the risk of bias assessments. We rated 13 of 32 trials that reported on deaths as being at probable or high risk of bias, primarily owing to issues with allocation concealment.
Risk of bias assessments for studies that reported deaths, using a modified version of the RoB 2.0 tool.
Network meta-analysis
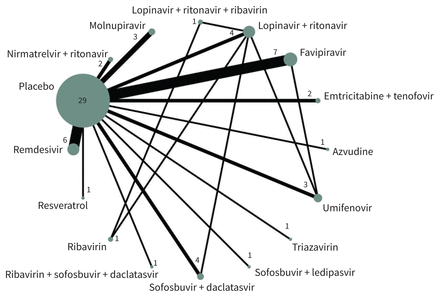
Our network meta-analysis included 40 trials, with 17 563 patients. We were unable to include 1 trial in the analysis because it did not report any of our outcomes of interest.51 Figure 3 presents the geometry of the network of trials that reported deaths and Appendix 1, Supplement 7 presents the network geometries for those that reported hospital admissions, mechanical ventilation and adverse events that led to stopping the drug.
Network diagram of antiviral drugs for COVID-19. Each sphere represents a drug or drug combination that has been tested in trials. The size of the sphere is proportional to the number of patients that have received that drug or drug combination, and the thickness of the connecting line is proportional to the number of trials.
Across all networks, most treatments were connected to standard care and placebo. Appendix 1, Supplement 8 presents treatment-splitting plots. Figure 4 and Appendix 1, Supplement 9 present results of the network meta-analyses, including relative risks from meta-analytic models. Both indices of heterogeneity (I2 and τ) were 0 across all outcomes. Appendix 1, Supplement 10 presents additional details related to heterogeneity for each network.
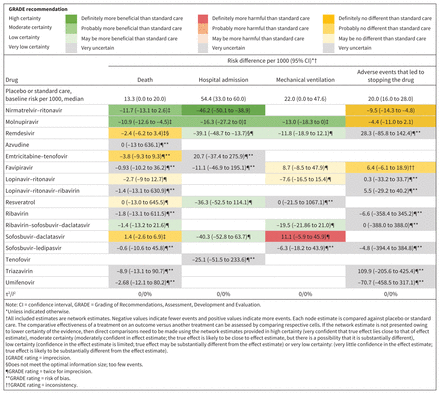
Network estimates of the effects of antiviral medications versus placebo or standard care, presented as risk difference per 1000 patients, with 95% confidence intervals (CIs).
Mortality
Thirty-two trials reported on deaths, including 10 837 patients and 291 deaths, with a median follow-up of 29 weeks.3,28,29,31,32,34–37,39,40,42–50,52–54,57–60,62–65,67 Based on median risk in the placebo and standard care group, we assumed a baseline risk of 13.3 deaths per 1000. Figure 5 presents the network forest plot for risk of death with treatment compared with standard care or placebo.
Forest plot presenting the network relative risk estimates for risk of death with antiviral drug versus placebo or standard care.
Compared with standard care or placebo, molnupiravir and nirmatrelvir–ritonavir each reduced risk of death with moderate certainty (10.9 fewer deaths per 1000, 95% CI 12.6 to 4.5 fewer for molnupiravir; 11.7 fewer deaths per 1000, 95% CI 13.1 fewer to 2.6 more). Remdesivir (2.4 fewer deaths per 1000, 95% CI 6.2 fewer to 3.4 more), sofosbuvir–daclatasvir (1.4 more deaths per 1000, 95% CI 2.6 fewer to 6.9 more) and emtricitabine–tenofovir (3.7 fewer deaths per 1000, 95% CI 9.3 fewer to 9.29 more) had no effect on risk of death, with moderate certainty.
Hospital admission
Ten trials reported on hospital admissions, including 5575 patients with 252 events, with a median follow-up of 21 weeks.3,30,35,39,42,48,53,55,56,67 Based on median risk in the placebo and standard care group, we assumed a risk of 54.4 hospital admissions per 1000.
Compared with standard care or placebo, nirmatrelvir–ritonavir reduced the risk of hospital admission (46.2 fewer admissions per 1000, 95% CI 50.1 to 38.9 fewer; high certainty), molnupiravir probably reduced risk of admission (16.3 fewer admissions per 1000, 95% CI 27.2 to 0 fewer; moderate certainty) and remdesivir may have reduced risk of admission (39.1 fewer admissions per 1000, 95% CI 48.7 to 13.7 fewer; low certainty).
Compared with molnupiravir, nirmatrelvir–ritonavir probably reduced risk of hospital admission (27.8 fewer admissions per 1000, 95% CI 32.8 to 18.3 fewer; moderate certainty).
Mechanical ventilation
Fourteen trials reported need for mechanical ventilation, including 3972 patients with 123 events, with a median follow-up of 21 weeks.29,31,32,34,35,44,45,48–50,56–59 Based on median risk in the placebo and standard care group, we assumed a baseline risk of 22 mechanical ventilation events per 1000.
Compared with standard care or placebo, molnupiravir probably reduced the need for mechanical ventilation (13 fewer events per 1000, 95% CI 18.3 to 0 fewer; moderate certainty) and remdesivir may have reduced mechanical ventilation (11.8 fewer events per 1000, 95% CI 18.9 fewer to 12.1 more; low certainty).
Sofosbuvir–daclatasvir probably increased the risk of mechanical ventilation compared with standard care or placebo (11.1 more events per 1000, 95% CI 5.9 fewer to 45.9 more; moderate certainty).
Adverse events that led to stopping the drug
Twenty-two trials reported on adverse events that led to stopping the drug, including 7465 patients and 190 events, with a median follow-up of 29 weeks.3,28,33–35,37,39,42–47,49,50,53,54,56,58,59,66,67 Based on median risk in the placebo and standard care group, we assumed a baseline risk of 20 events per 1000.
Compared with nirmatrelvir–ritonavir, molnupiravir had similar rates of adverse events (5.1 more events per 1000, 95% CI 3 fewer to 13.2 more; moderate certainty). Compared with standard care or placebo, molnupiravir (4.4 fewer events per 1000, 95% CI 11 fewer to 2.1 more; high certainty) and nirmatrelvir–ritonavir (9.5 fewer events per 1000, 95% CI 14.3 to 4.8 fewer; high certainty) did not increase adverse events that led to stopping the drug.
Subgroup analysis
For comparisons of molnupiravir, nirmatrelvir–ritonavir and remdesivir, we performed within-trial metaregressions of the effects of age, sex and mild versus moderate severity. For deaths, 2 remdesivir trials reported on subgroup data for age and sex but did not find evidence of subgroup effects.29,34 For hospital admissions, only 1 molnupiravir trial reported within-trial subgroup data and did not find evidence of a subgroup effect by disease severity, age or sex.35 We performed a between-trial subgroup analysis for risk of bias and did not find evidence that results are different between trials at low versus high risk of bias. Appendix 1, Supplement 11 presents the results of these analyses and Appendix 1, Supplement 12 presents pairwise comparisons for each outcome.
Interpretation
In our comprehensive systematic review and network meta-analysis addressing the comparative effectiveness of antiviral drugs for nonsevere COVID-19, we found that molnupiravir and nirmatrelvir–ritonavir probably reduce risk of death and hospital admission without increasing adverse events, and that nirmatrelvir–ritonavir is probably more effective than molnupiravir at reducing risk of hospital admission. Remdesivir may reduce risk of hospital admission and need for mechanical ventilation but with low certainty.
Despite limited availability of data on nirmatrelvir–ritonavir, evidence has consistently shown reductions in hospital admissions and deaths, and the certainty of evidence may increase with accumulating data.4,5,68
Our review has implications for guideline developers and health care systems. The United States Food and Drug Administration currently licenses molnupiravir for emergency use, a decision that has been criticized because of the drug’s potential mutagenic properties,68 which may increase the risk of cancer and contribute to the emergence of new SARS-CoV-2 variants.69 Our findings suggest that nirmatrelvir–ritonavir may be superior to molnupiravir for some outcomes, which has implications for organizations, such as the WHO, that are in the process of developing recommendations addressing molnupiravir and nirmatrelvir–ritonavir.1 Health care systems deciding on drug procurement and cost issues need to consider the relative efficacy of nirmatrelvir–ritonavir over molnupiravir.
Although we show evidence that nirmatrelvir–ritonavir is superior to molnupiravir for some outcomes, we have no evidence of the efficacy of combination therapy. Combination therapy may be promising, not only to improve outcomes for patients but also to reduce the likelihood of resistance. For example, combination antiretroviral drugs have been effective at reducing resistance in patients with HIV.70 This is particularly important given the nonlinear effects of communicable diseases.
Antiviral treatments for COVID-19 were first studied early in the pandemic, primarily with patients admitted to hospital with severe and critical COVID-19.71–73 We posit, however, that antiviral drugs are most useful in nonsevere disease, which is driven by viral proliferation, rather than in severe disease, which is primarily driven by an inflammatory response.74,75 Previous reviews addressing antiviral drugs for all disease severities have found little-to-no benefit. Because antiviral drugs may be most useful in nonsevere disease, evidence from this review addresses an important gap in evidence.
Recently, concerns have been raised about a rebound phenomenon with nirmatrelvir–ritonavir, whereby patients develop symptoms of COVID-19 after taking the drug. This was addressed in an advisory statement by the US Centers for Disease Control and Prevention on May 24, 2022, which recommended continued use of the drug. A recent study found that rebound occurred in 0.8% of patients, resulted in mild symptoms and did not require additional COVID-19 therapy.76 Further research is needed to identify reasons for rebound phenomena, their severity and their impact on health care systems.
The strengths of this systematic review and network meta-analysis include use of state-of-the-art methods to synthesize and appraise the evidence. Unlike other reviews that have addressed antiviral drugs for COVID-19, we focus on nonsevere patients — patients who are most likely to benefit from antiviral drugs.2
Unlike other treatment options for nonsevere COVID-19 such as monoclonal antibodies, antiviral drugs can be administered orally in an outpatient setting. Theoretical considerations suggest that antiviral drugs may be at lower risk of substantial changes in efficacy in emerging variants than monoclonal antibodies. For example, nirmatrelvir–ritonavir, a protease inhibitor, is likely to be effective in reducing replication of all SARS-CoV-2 variants that rely on proteases for the viral lifecycle. Conversely, the extracellular proteins that are targeted by monoclonal antibodies are subject to change across SARS-CoV-2 variants.77
Limitations
Our results are limited by a dearth of published data on nirmatrelvir–ritonavir, currently from only 2 trials (EPIC-HR and EPIC-SR), the results of which (EPIC-SR) were provided in a press release.3,67,68 The recently published EPIC-HR trial was terminated early and thus may be at risk of overestimating benefits.78 We also currently have insufficient evidence of the effectiveness of nirmatrelvir–ritonavir for patients who were fully vaccinated against SARS-CoV-2 or the effect of antiviral drugs among patients with the Omicron variant. Indeed, the lack of reporting of variant data is a limitation for all COVID-19 evidence synthesis. Results from our review for this antiviral are primarily driven by EPIC-HR, which included only patients who were at high risk of developing severe disease and patients who were unvaccinated against SARS-CoV-2.67 Although EPIC-SR — a trial that recruits patients at standard risk for severe disease — includes vaccinated patients, such patients were required to also have additional risk factors for severe disease. Similarly, data on molnupiravir came from only 3 trials, 2 of which had a combined sample size of fewer than 100 patients and 1 of which included nearly 1500 patients. Subgroup data on vaccinated patients from this trial are currently unpublished. To address such limitations, an update of this review can be done when new, potentially practice-changing evidence becomes available.
Although we were rigorous when reviewing citations, including reviewing large systematic reviews that addressed COVID-19 treatments, it is possible that we missed articles. The Epistemonikos database is relatively new but has been recently validated as a comprehensive source for COVID-19 articles.9 However, the use of automated tools and assessment by human reviewers can also lead to errors in the systematic review process. A further methodological limitation is that there were few head-to-head comparisons of active interventions. Detecting local incoherence requires both direct and indirect estimates; when few trials directly compare antiviral drugs, tests for local incoherence are less sensitive.
Estimates of absolute effects are dependent on the baseline risk, which may vary across populations. We encourage clinicians to consider the anticipated baseline risk in their own patients when applying this evidence.
Limited data exist on the safety of antiviral drugs (particularly nirmatrelvir–ritonavir and molnupiravir) for people who are pregnant and breastfeeding, who are usually excluded from trials, and on long-term follow-up for safety outcomes, such as mutagenicity of molnupiravir.3,35,46,67,79 No trials have evaluated the effectiveness of these antiviral drugs for pre- and postexposure prophylaxis.
Although we find compelling evidence supporting the efficacy of nirmatrelvir–ritonavir, ritonavir is an inhibitor of CYP3A4, an enzyme responsible for the metabolism of about half of all drugs, including dexamethasone. Clinicians must remain vigilant for potential drug interactions.80,81
Both nirmatrelvir–ritonavir and molnupiravir are expensive and in limited supply, making accessibility in low- and middle-income countries particularly difficult.
To assess the certainty of evidence, we used thresholds based on a survey of the authors; these are subjective and others may consider different magnitudes of effect important.
Finally, the evidence of how treatment with antiviral drugs affects the long-term sequelae of COVID-19, including long COVID-19, is unclear.
Conclusion
Molnupiravir and nirmatrelvir–ritonavir probably reduce risk of hospital admission and death among patients with nonsevere COVID-19. Compared with molnupiravir, nirmatrelvir–ritonavir probably reduces risk of hospital admission. Data from ongoing and future trials may improve the certainty of evidence and allow us to make stronger claims about the comparative efficacy of antiviral treatments.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Tyler Pitre and Dena Zeraatkar conceptualized and designed the study. Tyler Pitre, Gareth Leung, David Mikhail, Ellen Cusano and Faran Khalid contributed to data collection. Tyler Pitre performed analyses. Rebecca Van Alstine and Genevieve Chick contributed to data interpretation. Dena Zeraatkar is the study supervisor and supervised the analysis. Tyler Pitre drafted the manuscript, and all of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: None.
Data sharing: Data are freely available at https://osf.io/zbcf9. Further request can be made by contacting the corresponding author.
- Accepted June 23, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/